News & Press Releases
Advanced Spinal Reconstruction Procedure Now Available in Central Florida
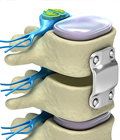
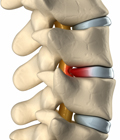
Total Disc Replacement (TDR) Technology Offers a Surgical Alternative to Spinal Fusions
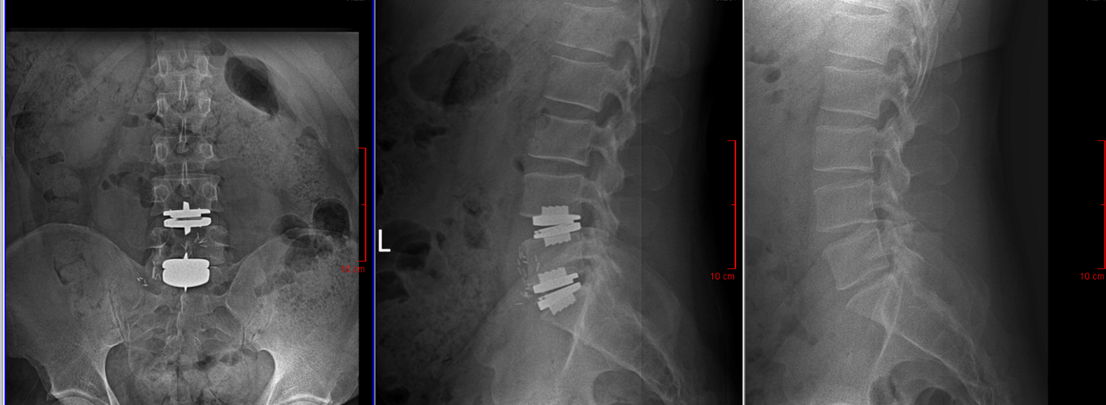
CELEBRATION, FLORIDA (March, 2023) - Dr. Maahir Haque, a Board Certified and Fellowship Trained Orthopedic Spine Surgeon with Spine Group Orlando at Celebration Orthopaedics is at the forefront of Motion Preservation and Minimally Invasive Spine Surgery in Central Florida. He is the area’s leader in Lumbar Total Disc Replacements (TDR) procedures and has performed dozens of these procedures, on patients who come from around the Southeastern United States. Dr. Haque recently performed his fifth multi-level Lumbar Disc Replacement.
Whereas lumbar disc replacement was previously only FDA approved for single-level problems, Centinel Spine’s clinically proven prodisc® L Total Disc Replacement (TDR) technology has now received FDA approval for two-level implantation. Many patients who previously were not candidates will now have access to this life-changing procedure. The procedure offers a surgical alternative to spinal fusions in the lumbar spine and works to relieve pain in patients suffering from degenerated spinal discs, while maintaining motion over the long term[1] at the diseased spinal segment, and reducing adjacent-level degeneration and re-operations[2].
As spine treatments continue to advance through innovative technologies and motion preserving techniques, patients are seeking out alternatives to fusions. Dr. Haque discussed his approach to spine care: “Most patients can recover a pain-free life with conservative care for their neck and back issues, however a small fraction require surgical treatment to relieve their pain. My primary goal has always been to provide the highest quality of care to our patients in the least invasive manner so that they may return to their functional lives as quickly and comfortably as possible. The Total Disc Replacement procedure is a minimally invasive spinal surgery that is used to treat degenerated and damaged discs, while preserving the natural structure and alignment of the spine and restoring fluid movement and flexibility.”
Dr. Haque went on to say, “Many patients are surprised to learn that lumbar disc replacement technology exists and that they are a candidate for the procedure. With the newly approved two-level indication for prodisc L, more of my patients will be able to benefit from disc replacement technology. By maintaining motion in the once-diseased segment of the spine through a minimally-invasive anterior approach, my patients benefit from a much faster recovery and return to active life, while decreasing the likelihood of developing degenerative changes at adjacent levels of the spine. Unlike many fusion patients, disc replacement patients can return to sports and work, without physical limitation or permanent activity restrictions.”
Disc replacements are durable, having been studied in active-duty military patients. 83% of active duty military returned to full duty after lumbar disc replacement, while only 67% of fusion patients returned to full duty3. Even years after surgery, a comparative five-year study showed a three times lower likelihood of adjacent level degeneration in those patients receiving the prodisc L total disc replacement versus those who received a fusion4. (Adjacent-level degeneration was characterized by a composite score including disc height loss, endplate sclerosis, osteophytes, and spondylolisthesis.)
The prodisc L system received one-level FDA approval in 2006 and since 2020 is the only total disc replacement device in the U.S. approved for two-level use in the lumbar spine. Recent expanded insurance coverage for lumbar TDR with the prodisc L device has contributed to the patient’s ability to have this procedure.
For today’s patient looking forward to a speedy recovery to an active life-style, disc replacement with prodisc L is a powerful treatment option worthy of consideration for appropriate patients.
For more information about Total Disc Replacement or other spine surgeries, please visit: www.spinegrouporlando.com or call 321-939-0222.
About Spine Group Orlando at Celebration Orthopaedics and Dr. Maahir Haque:
Dr. Maahir Haque is a Board Certified Orthopedic Surgeon, who is also Fellowship Trained in Spine Surgery. He specializes in minimally invasive spine surgery which uses tubular retractors and reduces the length of the incision to enable many patients to go home the same day for a faster and less painful recovery. Dr. Haque is able to truly tailor a patient’s treatment plan to his or her individual needs and goals. He is referred complex spine patients from across the State of Florida and other countries through Spine Group Orlando’s international program. Dr. Haque is trained in revision spine surgical procedures, complex reconstructive spinal surgeries and the most advanced motion-preserving procedures including artificial disc replacement. Dr. Haque is actively involved in the national and international spine surgery community and has published a number of peer-reviewed articles in spine surgical journals, including the leading journal Spine. He has given a number of podium presentations in a variety of meetings including at the prestigious Scoliosis Research Society. A devoted teacher, Dr. Haque was a clinical instructor at Brown University and currently holds a faculty appointment at the University of Central Florida. He continues to lecture in spine surgery topics and to instruct other surgeons in advanced spine surgical techniques.
Dr. Haque is a proud Floridian and is excited to be home after many years away. He currently resides in the Orlando area with his wife, who is a practicing cardiologist in the community. In his spare time, Dr. Haque loves playing golf and is a true fishing enthusiast, never missing a chance to take his children on the water.
Spine Group Orlando is a part of Celebration Ortho and is located at 2954 Mallory Circle, Suite 101, Celebration, Florida 34747.
About Centinel Spine, LLC:
Centinel Spine®, LLC is a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access. The company offers a continuum of trusted, brand-name, motion-preserving and fusion solutions backed by over 30 years of clinical success—providing the most robust and clinically-proven technology platforms in the world for total disc replacement (prodisc®) and Integrated Interbody™ fusion (STALIF®).
Centinel Spine continues to advance its pioneering culture and corporate mission to become a catalyst of change in the spine industry and alter the way spine surgery is perceived. Centinel Spine remains the only company with comprehensive motion-preserving and fusion solutions for both cervical and lumbar anterior column reconstruction.
For more information on Centinel Spine products and technologies, please visit the company’s website at www.CentinelSpine.com and connect on Twitter and Facebook.
[1] Zigler, J, Delamarter R, Five-year results of the prospective, randomized, multicenter, Food and Drug Administration investigational device exemption study of the prodisc L total disc replacement versus circumferential arthrodesis for the treatment of single-level degenerative disc disease, J Neurosurg Spine, 2012, 17:493-501.
2 Zigler J, Glenn J, Delamarter R, Five-year adjacent-level degenerative changes in patients with single-level disease treated using lumbar total disc replacement with prodisc L versus circumferential fusion, JNS, 17:504-511, 2012.
3 Tumialan, L.M., et al., Arthroplasty in the military: a preliminary experience with prodisc C and prodisc L. Neurosurgical focus, 2010. 28(5): p. E18
Maahir Haque, MD is recognized as a leader in the field of minimally invasive spine surgery. At Spine Group Orlando, Dr. Maahir Haque also provides second opinions for spine surgery and MRI reviews for those with back pain and neck pain. Dr. Haque emphasizes non-surgical options for back pain and neck pain where possible. This can include accessing a back pain specialist with expertise in pain-relieving spinal injections and spine therapists. Spine therapy can include back stretches that can be a future home remedy for back pain or neck pain. If spine surgery is necessary because of a herniated disc, spinal fracture, or spinal stenosis, Dr. Maahir Haque operates through tubular retractors that reduce the size of the incision, lessen blood loss, reduce time in the hospital, speed return to activity with a less painful recovery. This spine surgery expertise enables many patients to have outpatient spine surgery and be home the same day. Spine Group Orlando and Dr. Maahir Haque provides artificial disc replacement in the neck using the Mobi-C disc implant, the first FDA-approved disc for multiple levels in the neck. Prodisc-C is also used for artificial disc replacement in the cervical spine. Dr. Haque is also one of the few spine surgeons in Orlando, Florida to provide lumbar artificial disc replacement using the Prodisc-L artificial disc. Dr. Haque is also referred patients from across Orlando and north central Florida for artificial disc replacement surgery as an alternative to spinal fusion. Accordingly, Dr. Haque's patients travel from across north central Florida, including: Orlando; Jacksonville; Tallahassee; Lakeland; Gainesville; Tampa; Daytona Beach; and Cocoa Beach. The spine center, as a destination for medical tourism for some international patients from Mexico and the Caribbean, can provide recommendations to out-of-town patients on nearby hotels and tourist attractions. Dr. Haque is featured on the national site CentersforArtificialDisc.com as an author on the subject of artificial disc replacement for herniated discs in the neck. The Centers for Artificial Disc web site has content specific to disc replacement options and alternatives to spinal fusion. Click here to visit the Centers for Artificial Disc.